The pharmaceutical landscape is evolving quickly. Medical innovation and technological advancements are revolutionizing treatment options, yet a crucial element – quality management – remains stagnant. This stagnation manifests in global medicine shortages, compromising patient access to essential medications. Additionally, solving problems after the fact is costly and time-consuming, impacting the industry’s bottom line and its ability to innovate. Thus, a fundamental shift in quality management is necessary.
The Need to Redefine Compliance
Historically, both drug manufacturing and the quality function have been treated as cost centers in the industry. This perspective has led to practices like prioritizing lower-cost manufacturing sites over investments in innovative technologies. Similarly, the quality function and its leadership in the pharmaceutical industry have traditionally taken a reactive stance: focused solely on ensuring compliance with Good Manufacturing Practices (cGMPs) regulations.
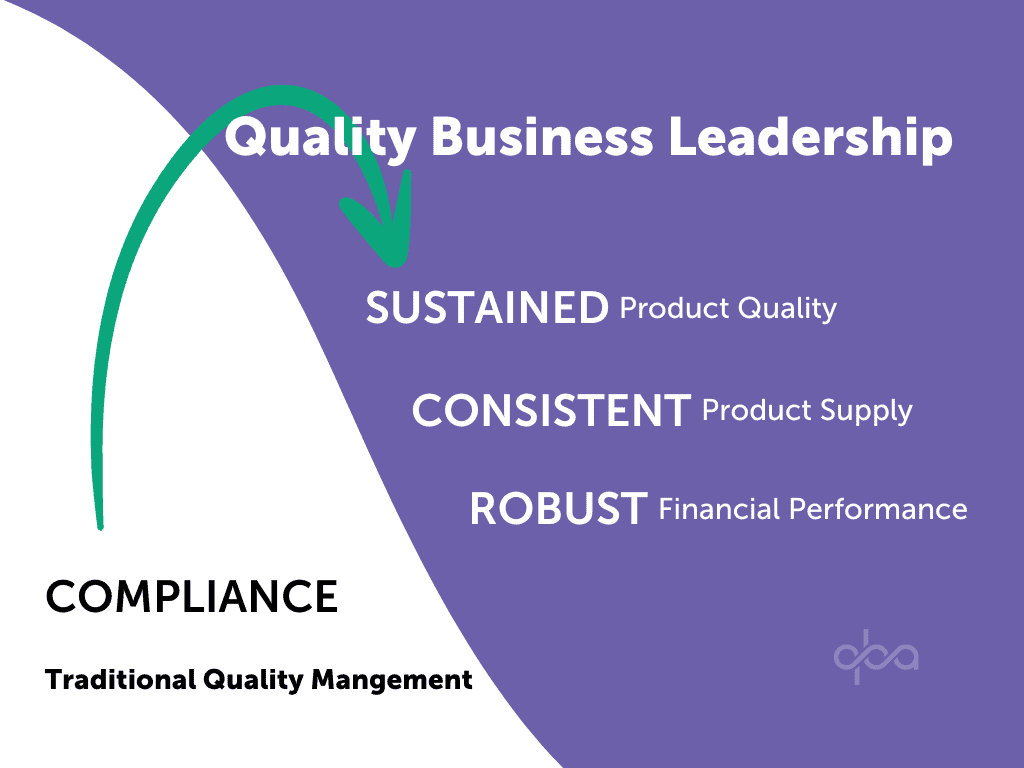
Setting the bar at ‘passing inspections with no observations’ equates to operating at the ‘floor’, the merely adequate quality level. This “compliance” mindset assumes that regulators set the standards, and companies simply need to meet them. It fails to acknowledge that true quality ownership lies with the individuals performing the work. This reactive stance not only disserves patients by leaving companies vulnerable to non-compliance but also misses the opportunity to leverage quality as a driver of financial performance. FDA has stated that they have low confidence in quality management of the pharmaceutical industry in their Quality Management Maturity (QMM) 2022 Whitepaper. These concerns are shared by regulators worldwide, who have stepped up efforts to ensure compliance. However, achieving an outstanding quality culture requires more than just regulatory cGMP compliance. It demands a proactive industry-led initiative, fostered through collaboration with stakeholders and spearheaded by strong Quality Business Leaders.
The pharmaceutical industry is embracing advancements like Advanced Therapy Medicinal Products (ATMPs) and general manufacturing modernization. However, many existing processes fall short of standards achieved in other industries. Quality leaders have a key role to play in ensuring that the company’s Pharmaceutical Quality System (PQS) acts as a springboard for new technologies, not a roadblock.
Overall, there is pressure on drug prices, and this pressure is likely to continue. This translates into the need for more cost-efficient ways of drug manufacturing while maintaining or even improving the level of product quality.
From Compliance to Strategy: The Rise of Quality Business Leadership (QBL)
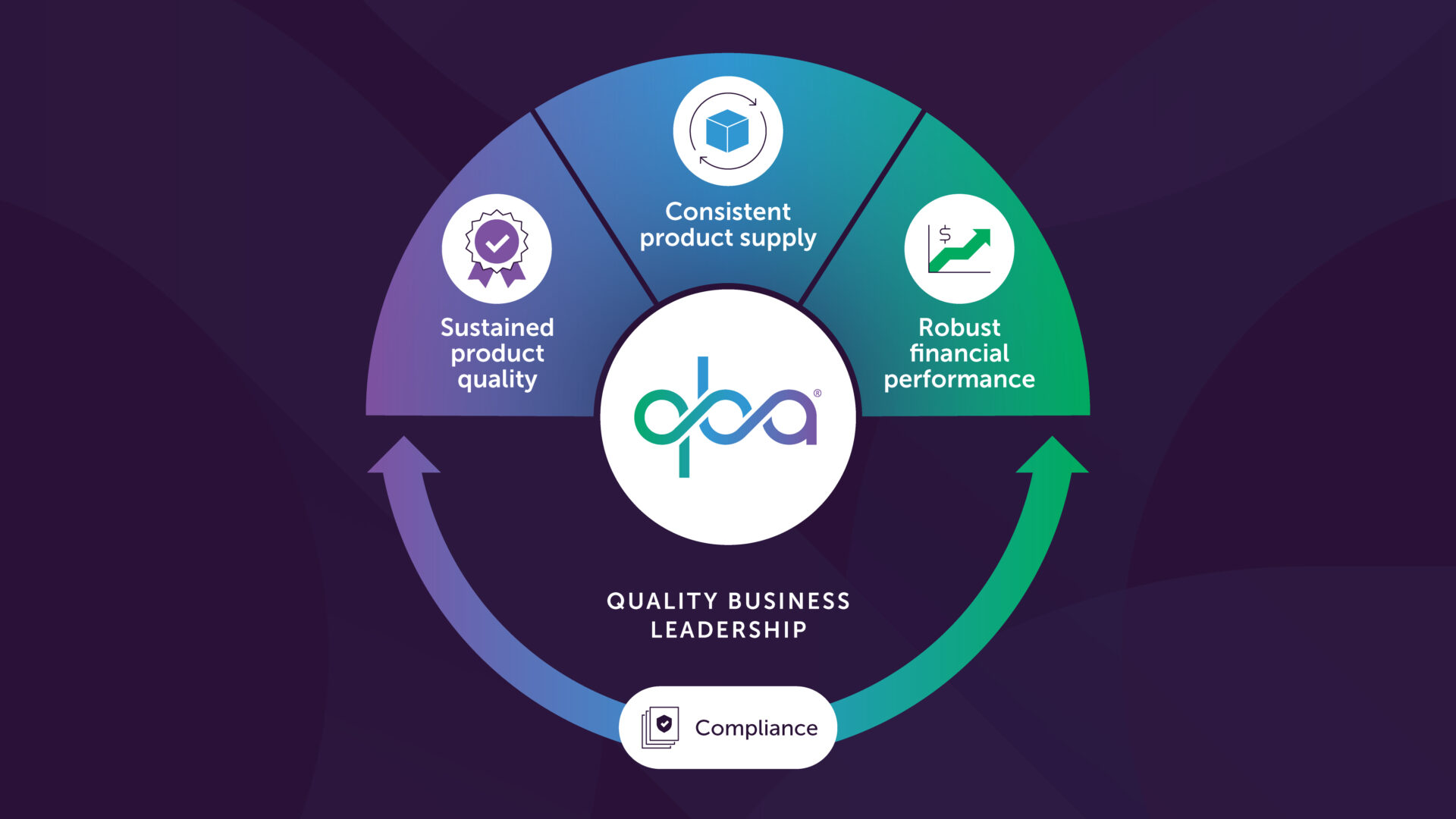
The pharmaceutical industry demands a new approach to quality management. We must move beyond a quality function solely focused on meeting regulations. Instead, quality leaders and the quality function need to become integrated strategic partners within the overall business. This new approach prioritizes not just sustained product quality, but also consistent product supply and robust financial performance. We call this transformative approach Quality Business Leadership (QBL).
The skillset needed to be proficient in QBL is very different from what is sufficient to ensure cGMP compliance. Six specific traits are required to become a great Quality Business Leader.
While the six traits may appear straightforward, mastering them requires advanced education, dedicated effort, and practice. New behaviors must be engrained in daily activities in a disciplined and structured fashion. This transition facilitates the Quality Function to a) turn quality from being seen as a cost center to a contributor to company financial performance, b) improve the reputation of the industry with fewer supply chain disruptions and consistent product quality, and c) enhance career opportunities of Quality leaders by developing them as key players within the organization.
The QBL Imperatives: A Call to Action
The need for Quality Business Leadership (QBL) was solidified at the inaugural Quality Business Leadership Summit held in Dublin, Ireland on May 1st, 2024. This gathering brought together over 100 leading figures from industry, academia, and regulatory agencies. The outcome was the creation of the Quality Business Leadership Manifesto (available below), a powerful call to action for senior leadership across Quality, Operations, and Regulatory Agencies.
The Manifesto signifies a crucial turning point. The change requires a united front, driven by both visionary leadership and a strong commitment from all levels within organizations. Regulators have a vital role to play in fostering this transition by adapting their approach to incentivize and support QBL implementation. Ultimately, the QBL will benefit all key stakeholders, garnering widespread societal support.
Quality Business Leadership Manifesto
Reimagining Quality Management in Pharma: A Call to Action
The pharmaceutical landscape is constantly evolving with medical breakthroughs and technological advancements. Yet, our quality management systems often struggle to keep pace, particularly in the face of concerning global medicines shortages.
Beyond Compliance: A New Definition of Success
Simply meeting cGMP regulations is no longer sufficient. We envision a future where quality management transcends regulatory requirements, becoming a strategic cornerstone for business growth.
Introducing the Quality Business Leader
This vision demands a new kind of leader: the Quality Business Leader. These leaders possess a deep understanding of both quality and financial principles, championing:
- Patient-Centric Focus: Every decision prioritizes patient safety and well-being. Rigorous benefit-risk analysis ensures patients have consistent access to safe, high-quality medications.
- Leadership by Ownership and Partnership: Quality becomes a shared mindset, nurtured at all levels across the organization. Leaders foster collaboration and a sense of ownership for quality excellence.
- Regulatory Collaboration: We advocate for a streamlined, global regulatory framework that encourages continuous improvement and innovation, including the implementation of new ideas and technologies.
- Streamlining for Efficiency: Quality systems and processes are simplified for enhanced effectiveness. Every step in the supply chain is optimized for the highest quality outcomes.
- Financial Advantage Through Quality: We achieve sustainable financial success while upholding the highest quality standards. Quality management becomes a driver of business prosperity.
The Quality Business Leader ensures not just sustainable product quality and supply, but also sustainable company financial performance.
A Call to Action
We are driven to disrupt the current state and forge a new path. The time for action is now! Achieving this vision demands unwavering commitment from everyone involved. Remember, every revolution begins as a vision, an idea taking shape in our minds before becoming reality. This is where it starts.
Let’s unleash the power of quality – are you in?
CEOs: Lead the Charge
The goal is audacious: transforming the entire pharmaceutical industry into Quality Business Leadership (QBL). We need your leadership to make it happen. Here’s how you can champion QBL:
- Become a QBL Advocate: Publicly endorse QBL by co-signing the Manifesto with your Chief Quality Officer (CQO). This sends a powerful message to your company and the industry.
- Invest in QBL: Allocate resources for QBL initiatives. This could include employee training in QBL principles or implementing new quality systems and processes.
- Champion Continuous Improvement: Initiate at least one Quality Improvement Project related to at least one of the QBL traits within your company, regardless of size. Showcase the value of quality and ignite a culture of improvement.
You can equip your quality leaders with the 10-week QBL Certification Program. Offered by the Technological University of Dublin in the fall of 2024, the program provides the necessary training and skills to excel in a QBL environment. Upon passing, the learners will be awarded Quality Business Administration Certification. Learn more about the Certification Program on the web page or by emailing Anders Vinther at anders@QBAleaders.com.
Whether you’re a CEO, Operations leader, regulator, or quality professional, your commitment matters. Sign the Manifesto to show your support for QBL. This can be done by making a comment in the LinkedIn post of this article or by emailing Anders Vinther at anders@QBAleaders.com.
This article has defined QBL, outlined the six leadership traits, and presented the QBL Manifesto. We’ve ignited a vital conversation about integrating quality into the core of the pharmaceutical business. By working together, we can ensure sustained product quality, secure supply for patients, and achieve long-term financial success for the industry.
The journey to QBL begins NOW.
Certificate in Quality Business Leadership
Next certification program starts 07 Oct 2026