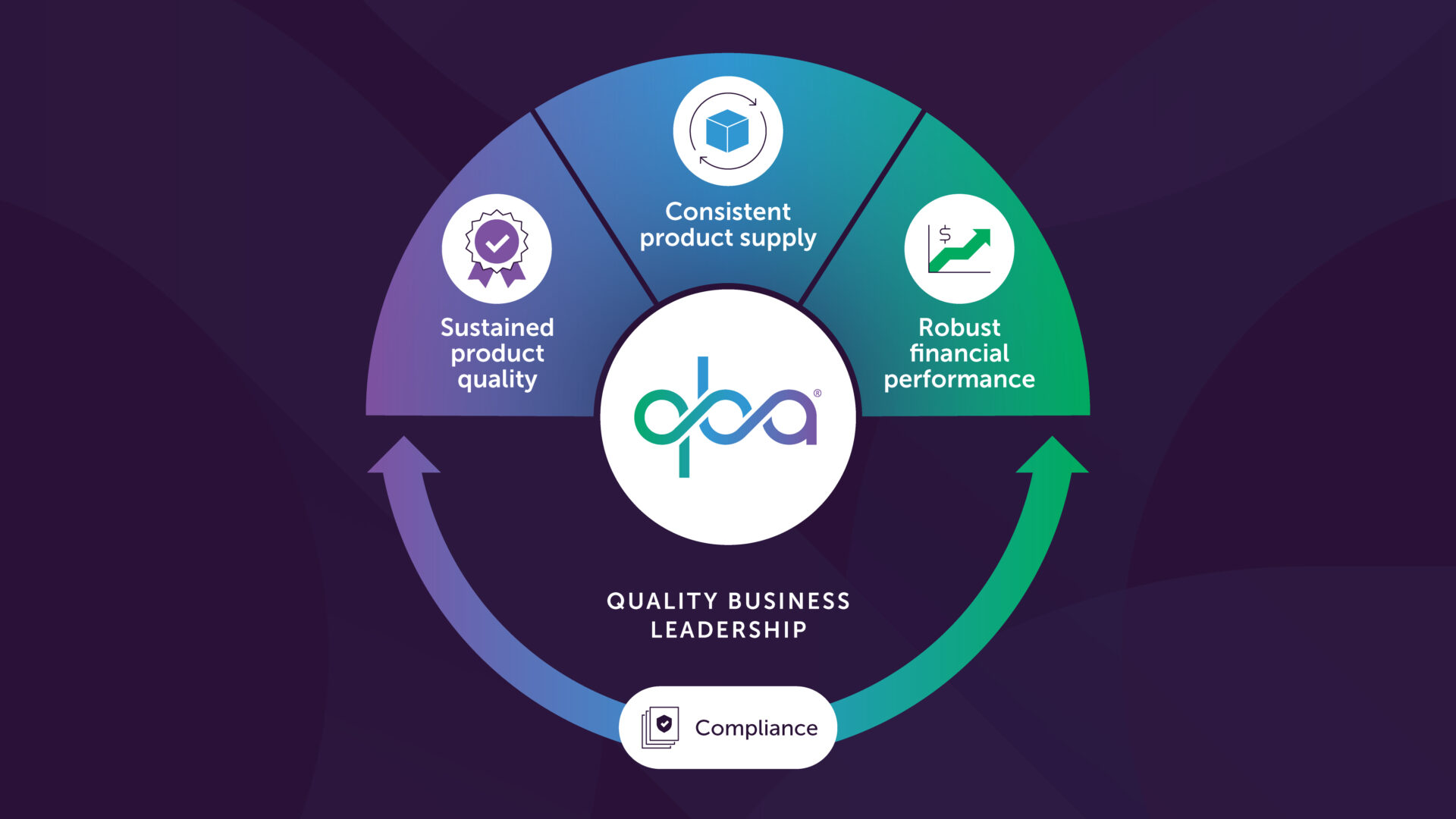
Quality Business Leadership (QBL) repositions the Quality Organization as a Strategic Business Partner that focuses on optimizing and innovating in the areas of robust financial performance, sustained product quality, and consistent product supply (see Figure 1). This significantly differs from the Traditional Quality Support Function that primarily ensures cGMP compliance.
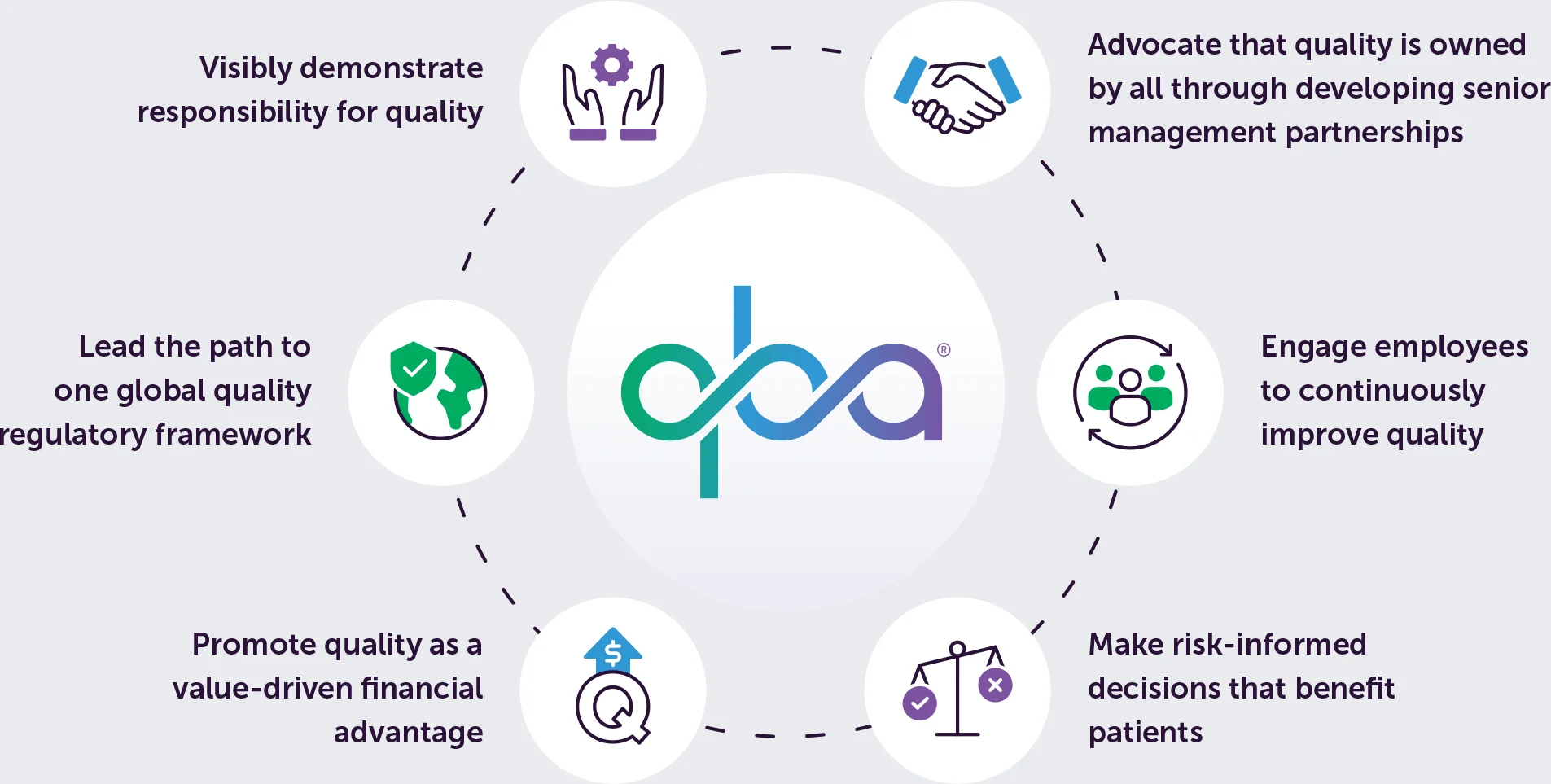
The Quality Business Leadership (QBL) Objectives
For the organization, the transition to QBL represents a widespread and fundamental shift in mindset on how the Quality Organization is perceived – moving from being seen as a reactive, necessary cost to an innovative, competitive, and value-driven asset.
Forward thinking leaders in organizations are now recognizing the value of integrating Quality as a Strategic Business Partner to the Executive Management and the Pharmaceutical Quality System (PQS) into the overall company business processes. They understand the necessity for Quality to be a priority owned across the organization, not just in one department, ensuring full alignment and support for success. They also realize the opportunity to differentiate themselves in the market as a trusted leader with fully optimized, highly efficient and effective systems that seamlessly serve patients in today’s uncertain world. In these organizations, the PQS is continuously simplified, accommodates to changes in supply, demand, business environment, and the implementation of new technologies.
For Quality professionals, transitioning to QBL requires unlearning inefficient and ineffective practices and mindsets while developing new skills and strategies. Quality Business Leaders master Six Key Traits and three QBL paradigm shifts (see Figure 2)
Six Key Traits
- Visibly demonstrate responsibility for quality
- Advocate that quality is owned by all through developing senior management partnership
- Engage employees to continuously improve quality
- Make risk-informed decisions that benefit patients
- Promote quality as a value-driven financial advantage
- Lead the path to one global quality regulatory framework
Three QBL Paradigms
- Focus on patients
- Lead more
- Think in systems
Traits and Paradigms of QBL
Trait: Visibly demonstrate responsibility for quality
Trait: Advocate that quality is owned by all through developing senior management partnership
Trait: Engage employees to continuously improve quality
Trait: Make risk-informed decisions that benefit patients
Trait: Promote quality as a value-driven financial advantage
Trait: Lead the path to one global quality regulatory framework
The Path from Traditional Support Function to Strategic Business Partner
Despite a stall in widespread action, senior executives have echoed Dr. Janet Woodcock’s aspirations for the future of Quality for decades. In 2004 she outlined FDA’s vision as “A flexible, agile pharmaceutical manufacturing sector that reliably produces high-quality drugs without extensive regulatory oversight.”
To achieve this vision, key changes must happen and the blueprint to get there is all within the Six Key Traits of QBL. QBL is a major catalyst for the necessary mindshifts and evolving processes and will prepare leaders and organizations for successfully navigating a complex and ever changing industry. If done effectively and collectively, organizations will recognize Quality professionals as key contributors within the corporate structure. Quality professionals will have also transitioned from a traditional compliance focused mindset (minimum expectations), to truly becoming Quality Business Leaders.
To kickstart the movement for change in our industry, 100 senior academia, regulatory agency and industry leaders met in May 2024 to develop a Quality Business Leadership Manifesto.
Quality Business Leadership Manifesto
Reimagining Quality Management in Pharma: A Call to Action
The pharmaceutical landscape is constantly evolving with medical breakthroughs and technological advancements. Yet, our quality management systems often struggle to keep pace, particularly in the face of concerning global medicines shortages.
Beyond Compliance: A New Definition of Success
Simply meeting cGMP regulations is no longer sufficient. We envision a future where quality management transcends regulatory requirements, becoming a strategic cornerstone for business growth.
Introducing the Quality Business Leader
This vision demands a new kind of leader: the Quality Business Leader. These leaders possess a deep understanding of both quality and financial principles, championing:
- Patient-Centric Focus: Every decision prioritizes patient safety and well-being. Rigorous benefit-risk analysis ensures patients have consistent access to safe, high-quality medications.
- Leadership by Ownership and Partnership: Quality becomes a shared mindset, nurtured at all levels across the organization. Leaders foster collaboration and a sense of ownership for quality excellence.
- Regulatory Collaboration: We advocate and lead the path to a streamlined, global regulatory framework that encourages continuous improvement and innovation, including the implementation of new ideas and technologies.
- Streamlining for Efficiency: Quality management systems and processes are simplified for enhanced effectiveness, agility, and full integration into the overall company business. Every step in the supply chain is optimized for the highest quality outcomes.
- Financial Advantage Through Quality: We achieve sustainable financial success while upholding the highest quality standards. Quality management becomes a value that drives business prosperity.
The Quality Organization transitions from a Support Function to a Business Partner and the Quality Business Leader ensures sustainable product quality, consistent product supply, and robust company financial performance.
A Call to Action
We are driven to disrupt the current state and forge a new path. The time for action is now! Achieving this vision demands changes to how we operate and an unwavering commitment from everyone involved. Remember, every revolution begins as a vision, an idea taking shape in our minds before becoming reality. This is where it starts.
Let’s unleash the power of quality – are you in?
The QBL Imperatives: A Call to Action, The Change
The Quality Business Leadership Manifesto is a powerful call to action for senior leadership across Quality, Operations, and Regulatory Agencies.
The Manifesto signifies a crucial turning point. The change requires a united front, driven by both visionary leadership and a strong commitment from all levels within organizations. Regulators have a vital role to play in fostering this transition by adapting their approach to incentivize and support QBL implementation. Ultimately, the QBL will benefit all key stakeholders, garnering widespread societal support.
The goal is audacious: transforming the entire pharmaceutical industry from Traditional Quality Support Function to Quality Business Partner through the implementation of Quality Business Leadership (QBL).
Implementing these first steps is a good start on the journey to QBL:
- Become a QBL Advocate: Publicly endorse the QBL Manifesto and share it within and outside your company. This sends a powerful message to your company and the industry.
- Invest in QBL education: Allocate resources for QBL initiatives. This could include employee training in QBL principles or implementing new quality systems and processes.
- Start developing and implementing a Quality Value Index (QVI) model to track costs that are revenue, cost of goods and labor impacting respectively. This will allow you to identify improvement opportunities.
- Add Financial Risk Numbers (FRNs) to your risk register. FRNs are calculated as severity x occurrence x cost of correction / cost of prevention. FRNs add a risk to business dimension to your already existing risk to patient risk ranking.
- Encourage and support volunteers to initiate quality improvement projects and celebrate successes.
- Have leaders outside Quality communicate progress on quality goals and metrics. This will help the process towards quality owned by all.
Learn more about QBL and QBL Education here or by emailing Anders Vinther at anders@QBAleaders.com or by using our contact form.
Note: Quality Business Administration LLC (QBA) is a company dedicated to the education of Quality Business Leaders and support of companies transforming Quality to become Strategic Business Partners. QBA services include the Certificate in Quality Business Leadership Program accredited by the Technological University of Dublin. The Program is offered under the Graduate Business School as part of the MBA program.