

Certificate in Quality Business Leadership
The only advanced certification program of its kind for learning the necessary leadership traits to become Quality Business Leaders.
Next certification program starts 07 Oct 2026
The Certificate in Quality Business Leadership (QBL) program is designed to transition Quality and other key leaders into Quality Business Leaders who make the Quality function a core company business competency.
The program develops the participants financial skills required to make quality and the quality system an engine for both quality and financial improvement; it teaches successful ways to engage senior leaders and employees across the company to take ownership for quality, and it develops patient focused, data and science decision-making weighing benefits and risks. The course aims at progressing the participants skills to master the 6 traits of successful quality business leaders and move from being mainly compliance focused to becoming focused on sustained product quality, consistent product supply and robust financial performance.
The QBA certification program is accredited by TU Dublin* and runs over a period of 10 weeks. Each week will include a 3 hour online virtual education session taught by experienced senior industry and regulatory agency leaders and 7 hours of preparation and homework.
The curriculum is built around the 6 Quality Business Leadership traits and includes theory, practical examples, cases studies, and practical application of the traits in your company. The class size will be kept small to facilitate class discussions.
Is the Certificate in Quality Business Leadership right for you?
The Certificate in Quality Business Leadership program is ideal for any Pharma Operations, Quality, and Regulatory Agency mid to senior-level leaders with 5-10 years experience and an ambition to advance their career and make Quality a fully integrated function.
Praise for QBL
Participants from the program had some great feedback about their experience.
Master the 6 Quality Business Leadership traits
The Certificate in Quality Business Leadership program will help you master the 6 Quality Business leadership traits:
- Visibly demonstrate responsibility for quality
- Advocate that quality is owned by all through developing senior management partnerships
- Engage employees to continuously improve quality
- Make patient focused, risk-informed decisions about quality
- Promote quality as a value-driven financial advantage
- Lead the path to one global quality regulatory framework

How does it work?
The Certificate in Quality Business Leadership program is a cohort-based course with live virtual classes coupled with online learning materials. You get:
- 10 live virtual weekly classes led by experienced senior industry and regulatory agency leaders
- Certificate in Quality Business Leadership with accreditation from TU Dublin
- More than 10 course modules
- Access to the course platform and companion community for 1 year
- Support and mentorship from the course instructors and guides
After earning the Certificate in Qualitiy Business Leadership, you will know how to...
✅ Develop comprehensive quality plans
Using the quality system to drive company financial improvements taking into account regulations, compliance, business strategy, and financial aspects✊ Mobilize employees
Within and outside the Quality organization to use their competencies to drive forward quality improvements📈 Leverage data, opinions, benefits, and risks
To make, justify, and communicate decisions which balance regulatory compliance with drug availability💸 Lead the design of the overall company strategy
with respect to transitioning from a culture of compliance with regulations to a holistic quality approach that delivers financial benefits
Benefits of the Certificate in Quality Business Leadership Program
For patients
- Quality Business Leaders that are focused on the patient
- Enhanced anticipation and mitigation of risks
- Consistent supply of quality products
For companies
- Tangible financial performance improvement
- Reputation improvement
- Many more employees engaged in quality continuous improvements
For the learner
- Improved career development in and outside Quality
- Greatly enhanced business acumen
- Expanding external network of peers
Program Lecturers and Facilitators
Experienced Teaching Team
The Certificate in Quality Business Leadership includes industry and regulatory agency experts with proven practical track record and experience in their field of expertise. The lecturers will bring new perspectives, cutting-edge insights, and approaches that can be practically applied for each of the six QBL traits.
 Founder & CEO of Quality Business Administration
Founder & CEO of Quality Business Administration Former Acting FDA Commissioner
Former Acting FDA Commissioner CEO at TeamRathgeber
CEO at TeamRathgeber Former CFO, Parenteral Drug Association
Former CFO, Parenteral Drug Association Head of Graduate Business School, TU Dublin
Head of Graduate Business School, TU Dublin Head of Part-time Education, School of Chemical and BioPharmaceutical Science, TU Dublin
Head of Part-time Education, School of Chemical and BioPharmaceutical Science, TU Dublin Retired CQO Pfizer
Retired CQO Pfizer QP and former CEO of IMB/HPRA and Biopharma Industry Quality Leader
QP and former CEO of IMB/HPRA and Biopharma Industry Quality Leader EVP, CTO Allogene. Senior Operations Executive
EVP, CTO Allogene. Senior Operations Executive Professional and certified coach, background in culture change and engagement
Professional and certified coach, background in culture change and engagement Professor, HPRA, Rapporteur ICH Q9(R1), expert in QRM
Professor, HPRA, Rapporteur ICH Q9(R1), expert in QRM Industry and Regulatory Advisor, PRST, TUDublin
Industry and Regulatory Advisor, PRST, TUDublin EMA, Deputy Chair IWG
EMA, Deputy Chair IWG Independent adviser on systems transformational change and leadership
Independent adviser on systems transformational change and leadership
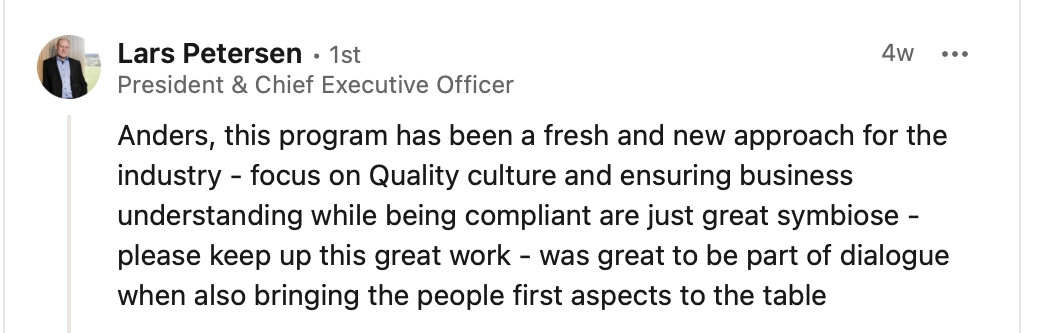
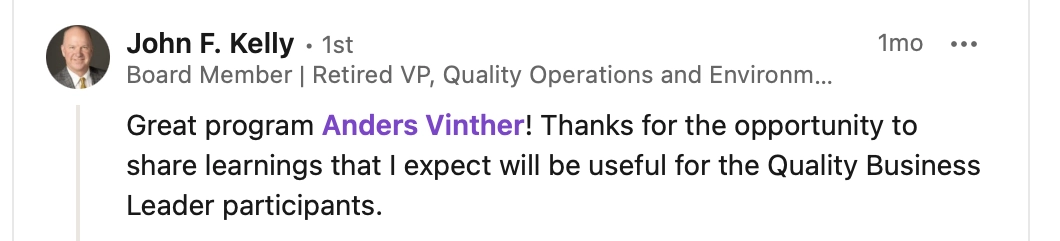
QBL is the way to integrate the Quality Function into the overall company business
Industry leaders agree...


Accredited by TU Dublin

This program is a 5 ECTS (Credits) minor award placed at Level 9 on the Irish National Framework of Qualifications (NFQ). It is offered by Technological University Dublin (TU Dublin), through the Graduate Business School (GBS), in partnership with QBA. It forms part of an emerging portfolio of Leadership in Life Sciences programmes co-developed by the GBS and TU Dublin’s School of Chemical and Biopharmaceutical Sciences. These programmes are specifically designed to support leadership development and enhance business and commercial acumen within the Pharmaceutical and broader Lifesciences sectors.
The GBS is one of 6 Schools within the Faculty of Business. An internationally accredited centre of postgraduate and executive education, the GBS is ranked in the top 2% of Global Business Schools. The School is home to over 2,000+ learners and is a trusted educational partner to leading Irish and international organisations. The wider Faculty of Business, Ireland’s largest Business Faculty, is home to over 6,500 of TU Dublin’s 28,000 students and operates across 3 campuses in Dublin – Aungier St, Tallaght and Blanchardstown.
Program Overview
A high-level overview of what the program covers week by week.
Note: topics and lecturers are subject to some slight modifications
Week 01 – Taking a Stand for Pharmaceutical Quality; Introduction to QBL
- Course introduction and overview / Introduction to Facilitators / Expectations
- Why we need Quality Business Leadership
- Introduction to six traits of QBL & the transition needed
- Quality Operating in headwinds. Quality’s role and focus today
Week 02 – Promote Quality As A Value-Driven Financial Advantage
This week we start talking about the trait promote quality as a value-driven financial advantage. We cover financial statements, cost management, linking quality to financial performance, Quality Value Index (QVI) models, and introduce Financial Risk Numbers (FRN).
Week 03 – Promote Quality As A Value-Driven Financial Advantage
This week we continue with the trait promote quality as a value-driven financial advantage.
Week 04 – Visibly Demonstrate Responsibility for Quality
Week 05 – Culture and Engagement
Week 06 – Culture and Engagement
Week 07 – Quality Owned by All
Week 08 – Risk-Informed Decisions
Decision Making 1
- Quality decision making authority, challenges in decision making
- QR9(R1), RBDM, decision making models, benefit/risk patient centric
- Decision making and prioritization, case studies 1
Week 09 – Risk-Informed Decisions
Decision Making 2
- Decision making case studies 2, communication of difficult decisions to authorities
- Working with regulatory authorities when good and when bad message
Week 10 – Lead The Path To One Regulatory Framework
Recap and Next Steps
- Summary of 6 traits, learnings, checklists and metrics
- Sharing of project plans by learners
- Conclusions and next steps
Partnership between TU Dublin and QBA
Aligned Visions

Technological University Dublin (TU Dublin), Ireland’s first Technological University, offers a wide range of programs from foundation to doctoral levels. Formed by merging three institutes of technology, TU Dublin is the second-largest third-level institution in Ireland, with over 28,000 students. The university emphasizes career-focused education and academic excellence in science, arts, business, engineering, and technology to develop future leaders.
QBA’s Quality Business Leadership program is designed to transform Quality Managers into Quality Business Leaders, benefiting patients, companies, and the managers’ careers. Although new, QBA leverages extensive experience to shift the Quality function from a cost center to a value generator.
Anders Vinther, QBA’s Founder and CEO, is involved with the Pharmaceutical Regulatory Science Team (PRST) and the 1VQ for PAC Initiative, collaborates with the top 25 pharma companies to simplify global Post Approval Change regulations. This partnership builds on Ireland’s unique environment of collaboration between regulatory agencies, academia, and industry, aiming to foster innovative Quality Business Leadership.
Certificate in Quality Business Leadership
Next certification program starts 07 Oct 2026